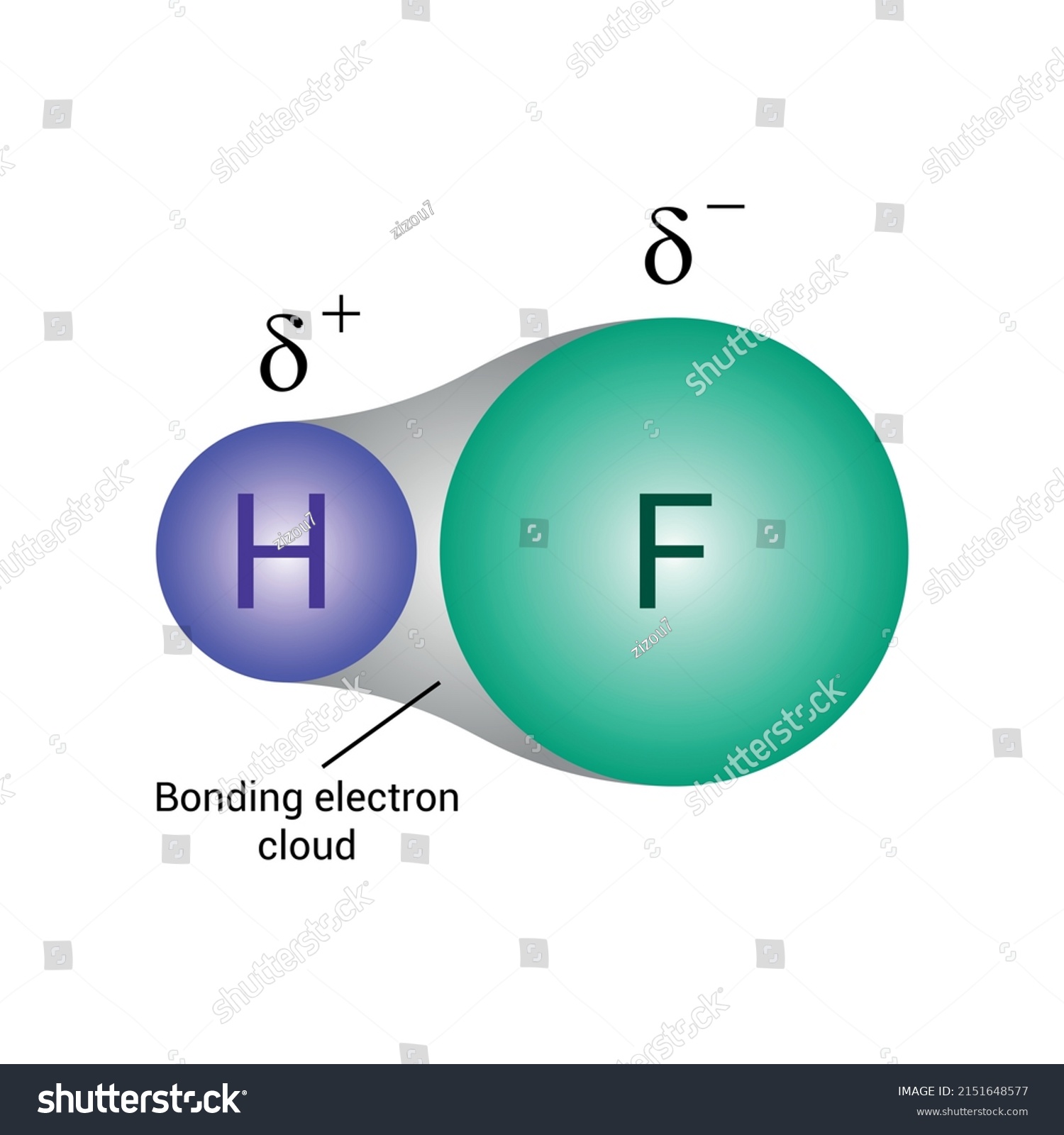
Hydrofluoric acid has a highly polar covalent bond due to the significant difference in electronegativity between hydrogen and fluorine. Fluorine being more electronegative. This systematic analysis explains the unique properties of this molecule; To determine whether fluorous acid is polar or nonpolar, we can examine it from three key perspectives: Molecular geometry, dipole moment, and electronegativity.
This is due to the high. Hydrogen fluoride (hf) is a fascinating chemical compound that exhibits a unique duality in its behavior. Unlike many other acids, hf can act as both an acid and a base, depending on the. Hydrofluoric acid is unique due to its ability to dissolve glass and metals, a property attributed to its polarity and reactivity. Unlike stronger acids, hf is considered a weak acid. Explain how the polarity of the hf molecule is related to its molecular structure. The high electronegativity difference between hydrogen and fluorine results in a very polar covalent. When vapours of hydrogen fluoride are dissolved in water, the resulting aqueous solution is known as hydrofluoric acid. It is a gaseous compound or dry liquid obtained by.
Indepth Look At Danny Brown In Chicago A Musical Odyssey
Biggie Whats Beef Lyrics The Impact And Meaning
Air Jordan Legacy 312 Low The Perfect Blend Of Style And Comfort